Year 2049 is the weekly newsletter that discusses the impactful innovations, discoveries, and research shaping our future.
If this was forwarded to you, subscribe for free to get a new story in your inbox every Friday.
Today’s Edition
Comic: The overthinking mosquito
Story: Malaria
A brief history of malaria
The current state of malaria
The new fight against malaria: gene editing, vaccines, and more
Video: A drone tour of the ITER fusion reactor
Comic
Story: Malaria
A brief history of malaria
Malaria is a disease we’ve been dealing with for thousands of years. Traces of the malaria parasite have been found in the remains of Egyptian mummies. Hippocrates described the fevers caused by malaria in Ancient Greece. The mosquito-filled Pontine Marshes protected Ancient Rome from invaders.
Back then, we thought the disease was caused by people breathing “bad air”, or “mal aria”. The relationship between mosquitoes and malaria was unknown.
Plasmodium falciparum, the deadliest form of malaria, was introduced by a new breed of mosquitoes around the 5th century. Some historians speculate that P. falciparum played a key role in the fall of the Roman Empire.
The mosquito has played a greater role in shaping our story than any other animal.
– Timothy Winegard, in his book “The Mosquito”
It wasn’t until 1897 that we understood that mosquitoes transmitted malaria. Sir Ronald Ross, a British doctor based in India, found the malaria parasite in the blood of Anopheles mosquitoes which proved a hypothesis that was first put forward by his predecessor Alphonse Laveran.
The current state of malaria

Today, more than 40% of the world’s population lives in areas at risk of malaria transmission. An estimated 627,000 people died from the parasite in 2020: 96% of the victims lived in Africa and 80% were children under 5 years of age.

Malaria cases vanished in rich countries decades ago as a result of attacking mosquito breeding grounds with insecticides like DDT and providing treatments and medications using quinine (a compound derived from the bark of cinchona trees found in South America).
While the overall number of deaths has been reduced over the past few decades, progress seems to be stagnating because evolution has caught up: malaria-infected mosquitoes have evolved and become more resistant to insecticides and antimalarial drugs. Meanwhile, insecticides like DDT have been proven to be harmful to the environment and our food chain. And finding new drugs that can resist malaria requires significant time, effort, and monitoring.
The new fight against malaria
These challenges have encouraged scientists to explore different solutions. The London School of Hygiene and Tropical Medicine estimates that malaria deaths can be cut by 75% by 2030 with the help of:
Gene-edited mosquitoes
Vaccines
Other measures like insecticide-treated nets (ITN)
This would avert an estimated 19.4 million deaths by 2050 (The Economist).

Let’s explore these solutions in more detail.
#1: Gene editing
Malaria is a disease transmitted by infected female Anopheles mosquitoes. With the latest advancements in genetic sequencing and editing, scientists are exploring if genetic modifications can be made to prevent malaria transmission.
The research alliance Target Malaria, which is supported by the Bill & Melinda Gates Foundation, wants to make genetic modifications to shrink the overall population of malaria-carrying mosquitoes. To do that, they want to create a “gene drive” to spread favourable genes to mosquito offspring.
More specifically, they want to:
Make female mosquitoes infertile so they don’t pass on the parasite to their offspring.
Make female mosquitoes have more male offspring since they don’t carry the parasite.
A research group at Imperial College London, a member of Target Alliance, successfully suppressed populations of malaria-carrying mosquitoes within 7-11 generations. The experiment was run over an entire year in large indoor cages that mimicked natural environments.
Risks and challenges: In a lab environment, gene editing can show promising results but it’s hard to anticipate unintended side effects. Even if we create malaria-free mosquitoes or suppress the population, the downstream effects this may have on local ecosystems are unknown and difficult to simulate. After all, mosquitoes are food for other animals including fish, turtles, dragonflies, birds, and bats.
Rigorous research, testing, and regulation are still needed before gene-edited mosquitoes can be released in real-world environments.
#2: Vaccines
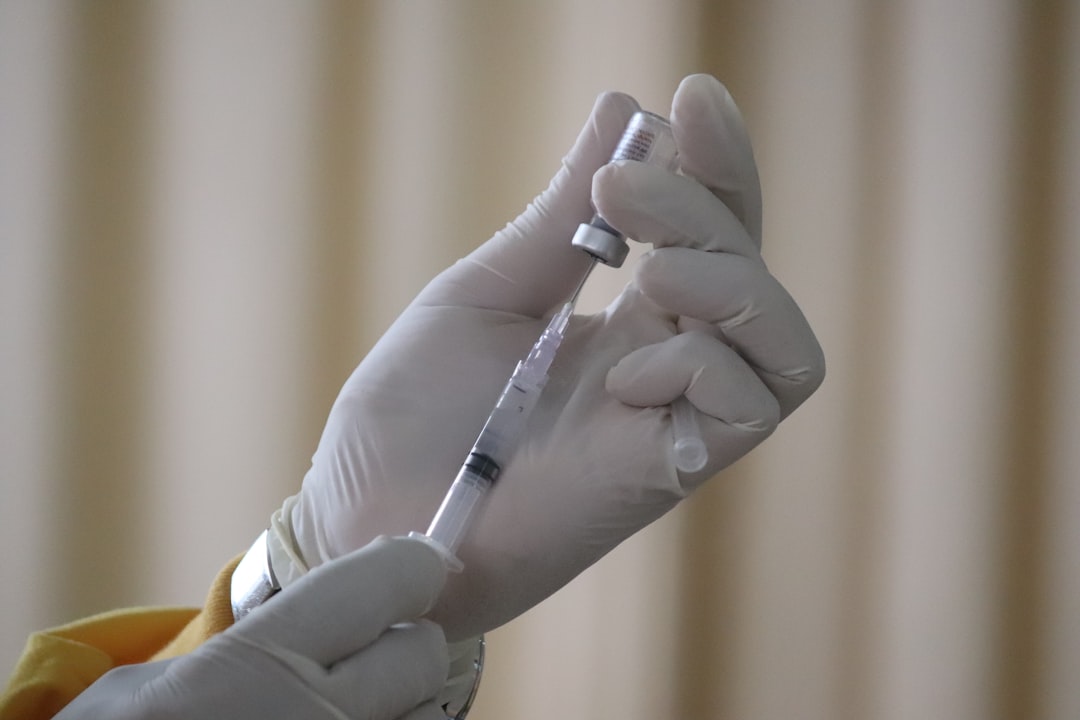
The first malaria vaccine was approved by the WHO last October. The vaccine, produced by GlaxoSmithKline, reduced severe malaria cases by 30% among 800,000 infants in Ghana, Kenya, and Malawi. The other important achievement is that it was proven to be safe for use.
Malaria vaccines have been so hard to make because P. falciparum is a parasite with a genome that is 1000 times larger than a virus like COVID-19. GSK’s vaccine, known as RTS,S or Mosquirix, is the result of 30 years of research.
GSK has committed to providing 15 million annual doses at no more than 5% above the cost of production. Infants were given four doses of the RTS,S vaccine so this annual commitment would be enough for 3.75 million infants (it’s unclear how the dosage would differ for adults). To put things in perspective, there were 241 million cases of malaria in 2020.
BioNTech and AstraZeneca are also developing malaria vaccines, but they’re still in clinical trials. A year-long trial of the AstraZeneca vaccine on 450 children in Burkina Faso showed up to 77% efficacy (Reuters).
#3: Other measures
The latest malaria report from the London School of Hygiene and Tropical Medicine discusses other effective solutions to tackle malaria:
Improving housing: Metal or tiled roofs along with concrete or brick walls reduce the odds of malaria infection by 14% compared to traditional housing.
Insecticides: long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS) are other ways of protecting homes from malaria. However, the increasing resistance to insecticides poses a challenge.
Chemoprevention: Seasonal malaria chemoprevention (SMC) for young children has been rolled out across 12 African countries to reduce infection rates. This method has been proven to be highly effective, but there’s the risk of mosquitoes becoming more resistant to these drugs.
Final thoughts
Malaria is a complex parasite and there’s no silver bullet to eradicate it. It’s clear that we need a combination of solutions to prevent infection and deaths, especially when the grand majority of victims are under 5 years of age.
Many of the solutions discussed will go through more research and clinical trials, so I’ll make sure to provide any updates about them in future editions of Year 2049.
Deep dive
If you enjoyed today’s story, I’ve compiled some additional links to satisfy your curiosity:
Bad air, amulets and mosquitoes: 2,000 years of changing perspectives on malaria (BMC)
The latest research report from the London School of Hygiene & Tropical Medicine (LSHTM)
Malarial mosquitoes suppressed in experiments that mimic natural environments (Imperial College)
Malaria-preventing bed nets save children’s lives—with impacts that can last for decades (Science)
Squashing malaria could save as many lives as covid-19 has taken (The Economist)
Video: ITER Fusion Reactor
I recently spoke about ITER, the world’s largest fusion reactor, in the Fusion Power episode. The organization just released the latest drone footage of the fusion reactor they’re building, which is set to be completed in 2025.
Previous episodes you might enjoy
🧬 CRISPR-Cas9 gene editing’s potential in healthcare
🍔 Miso Robotics wants to automate fast food
🚁 eVTOLs: the future of air transport?
You can also check out all previous Year 2049 editions to learn about other innovations that are shaping all areas of life.
How would you rate this week's edition?










Good job, Fawzi.
It would be good to resolve the scourge of malaria and your report today is encouraging in that regard
The gene editing approach can well be expected to be achievable given today’s advances in science. However the offsetting risks of unknown down stream effects on nature are potentially huge and too big a price to pay.
I’m therefore more inclined towards the vaccine and other methods approaches.
While these may be more difficult and time-consuming to implement than gene editing, the avoidance of the downside risks of the gene approach make that extra effort worthwhile